Invisible to the eye but impactful in its presence, sulfur dioxide (SO₂) is a chemical compound that we encounter more often than we might think. It occurs naturally through volcanic activity but is mostly manmade in industrial manufacturing and burning of fossil fuel. In high enough concentrations it is toxic and can be recognized by its very characteristic smell of burned matches. Incorrect handling of sulfur dioxide emission can pose serious threats to humans and the environment.
Chemical Composition of SO₂
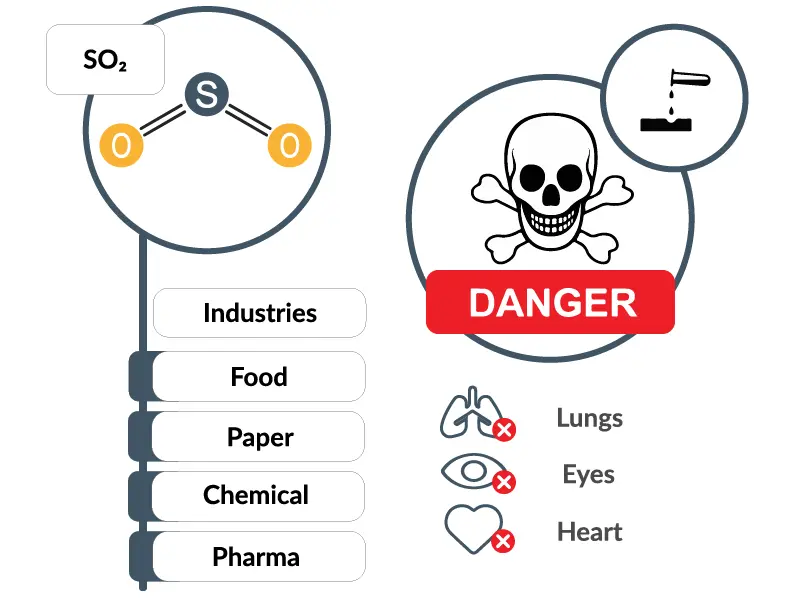
Sulfur dioxide is a colorless gas with a strong odor. It is a highly reactive substance and has a significant role in many industries like Food, Paper and Chemical. More details about its use cases later. Sulfur dioxide (SO₂) is a chemical compound consisting of one sulfur (S) atom and two oxygen (O) atoms bonded together. Furthermore, it is a corrosive and acutely toxic compound that can condense into liquid under high pressure. It is soluble in water and can be oxidized with airborne water particles to form sulfuric acid.

Industries that use Sulfur dioxide
In some industries sulfur dioxide can be a key component in the manufacturing process of some essential products. With these processes emissions can be emitted into the environment. It can react with other compounds in the atmosphere to form fine particles, which can be dangerous for human health and the environment. Some of the industries that combat with this challenge and their use case are listed below:
- Food and Beverage Industry:
- Preservative: Sulfur dioxide is used as a preservative in foods like dried fruits, fruit juices, and wine. It helps prevent the growth of microorganisms and extends the shelf life of these products.
- Antioxidant: It acts as an antioxidant in certain foods, helping to maintain the color, flavor, and quality of products like canned vegetables and fruit-based products.
- Paper Industry:
- Sulfur dioxide is used in the paper manufacturing process to bleach wood pulp and remove lignin (organic polymers). This helps produce high-quality, white paper products.
- Chemical Industry:
- Sulfur dioxide is a precursor to the production of sulfuric acid (H₂SO₄), a widely used industrial chemical. Sulfuric acid is essential for various processes, like the production of fertilizer and batteries.
- Reducing agent: In certain dyeing processes for textiles, it helps to fix colors onto fabrics.
- Pharmaceutical Industry:
- Sulfur dioxide can be used in some pharmaceutical processes, such as chemical synthesis and sterilization.
Challenges of SO2 in air filtration

Dangers of Sulfur dioxide
Sulfur Dioxide is known to irritate skin, eyes, nose, throat, and lungs in small concentrations. When concentrations are high it causes inflammation and irritation of the respiratory system. Symptoms can be pain while inhaling deeply, coughing, throat irritation and breathing difficulties. Especially during heavy physical activity these problems become apparent. It can cause heart disease but also lung problems like asthma. Older people, children and people with a weakened immune system are especially sensitive to sulfur dioxide.
Regulations
Emission regulations are strict on sulfur dioxide. Industries that emit sulfur dioxide need to obtain permits and report regularly on their emissions data. Both European and American laws state there is a limit to the amount that can be emitted to the environment. Because of the chemical complexity of sulfur dioxide, it can be hard to extract at the source. Therefore, many factories rely on exhaust air treatment end of pipe to reduce the emission of sulfur dioxide.
Solutions to effectively handle SO2
| Dry Scrubbers | |
|---|---|
| Positive | Negative |
| Low concentration of SO2 | High maintenance |
| No wastewater handling | Needs to be a dry exhaust gas (contain less than 0.65 liters of condensates per 50 cubic meter of produced gas) |
| Wet Scrubbers | |
|---|---|
| Positive | Negative |
| Can remove different kinds of pollutants, like SO2 | Wastewater handling |
| High temperatures and volumes | General large installations |
| High energy consumption | |
| Chemical Scrubbers | |
|---|---|
| Positive | Negative |
| Highly efficient for SO2 | Requires careful selection of chemical reagent |
| Converts SO2 to a less harmful compounds | Chemical reaction may create different waste products |
| Can be integrated with other air emission control stages | Can involve operational complexity |
| Desulfurization Units | |
|---|---|
| Positive | Negative |
| Concentrations higher than 90% | Wastewater handling |
| High temperatures and volumes | High energy consumption |
| General large installations | |
| Mainly effective for SO2 | |



